10 Facts about Boyle’s Law
If you like to study about science, you have to check Facts about Boyle’s Law. It talks about the experimental gas law. People like to call the Boyle’s law as Mariotte’s law or Boyle–Mariotte law. Have you ever learned about this law before? When you study about Boyle’s law, you will know how the pressure of the gas reduces when the volume of gas increases. Let’s find out more interesting facts about Boyle’s Law below:
Facts about Boyle’s Law 1: the volume and pressure
When you study about Boyle’s law, you will know the relationship between the volume and pressure of gas. Richard Towneley and Henry Power were considered as the first persons who noted the relationship between volume and pressure of gas.
Facts about Boyle’s Law 2: the discovery of Robert Boyle
Robert Boyle had his experiment to confirm the relationship between pressure and volume in his published work.
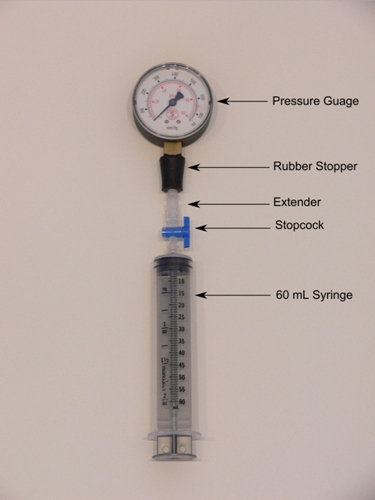
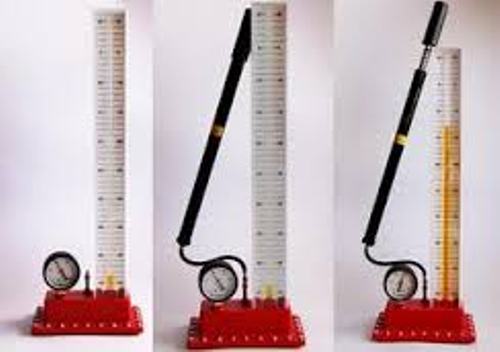
Facts about Boyle’s Law 3: the experimental apparatus
When Boyle tries to confirm the relationship between pressure and volume, he used a special experimental apparatus. The one who built the apparatus was Robert Hooke.
Facts about Boyle’s Law 4: the base of Boyle’s experiment
The experiment of Robert Boyle is based on air. Boyle rejected the idea that air was one of the four elements. He believed that air was an important element in life. Therefore, he elaborated his view by describing the plants which lived without air.
Facts about Boyle’s Law 5: the apparatus and materials
The experimental apparatus of Boyle was made in a closed J shaped Tub. One side of the tube was filled with mercury to find out the air pressure. The different amount of mercury was filled inside the tub to find out the proportional amount of pressure and volume.
Facts about Boyle’s Law 6: Edme Mariotte
In 1679, Edme Mariotte was a French physicist also established the same law. However, in 1662, Boyle had published his experiment. Therefore, this Boyle’s law is also called Boyle–Mariotte law or Mariotte’s law. Get facts about Alessandro Volta here.
Facts about Boyle’s Law 7: the inverse relationship
The inverse relationship is identified in Boyle’s law. But you have to make sure that it is kept in constant temperature.
Facts about Boyle’s Law 8: the decreased and increased pressure
If the pressure of gas decreases, the volume increases and vice versa.
Facts about Boyle’s Law 9: the amount of volume and pressure
If the volume is doubled, the pressure of gas is halved and vice versa. Get facts about air resistance here.
Facts about Boyle’s Law 10: the characteristics of gasses
When the gases are in moderate temperature and pressure, they act like ideal gases.
Do you have any opinion on facts about Boyle’s law?